It transfers genetic information form to DNA to ribosomes, a specialized structure, or organelle, which decodes genetic information into a protein.
Structure of mRNA
Post the onset of the COVID-19 pandemic mRNA has emerged as promising therapeutic tool in modern healthcare industry. With the help of genetic engineering, synthetic mRNAs can express proteins, as they structurally resemble a natural mRNA. Presently, more than 195 mRNA therapeutics / mRNA vaccines are under development or commercialized for the treatment of a variety of indications. As a result, there is an evident increase in the demand for mRNA manufacturing capacity.
Application of Synthetic mRNA
Several studies conducted by healthcare companies and research institutions have demonstrated the potential of mRNA.
Contract Manufacturing in mRNA Synthesis AND Manufacturing Service Domain
The synthesis and manufacturing process of mRNA-based therapeutics / vaccines is complex and associated with several challenges. The production of such drug products requires skilled labor, stringent manufacturing protocols and specialized expertise. Further, the biggest challenge in this domain is the purification which involve the use of hazardous solvents / materials and stability of drug products (thereby, requiring specialized facilities and cold chain transportation). Additionally, they also require appropriate drug delivery systems to efficiently administer the intervention (in a manner that they can avoid degradation by cellular endonucleases). However, because of such operational and technical challenges associated with the production of RNA-based products innovators in the biopharmaceutical industry are increasingly relying on the contract service providers.
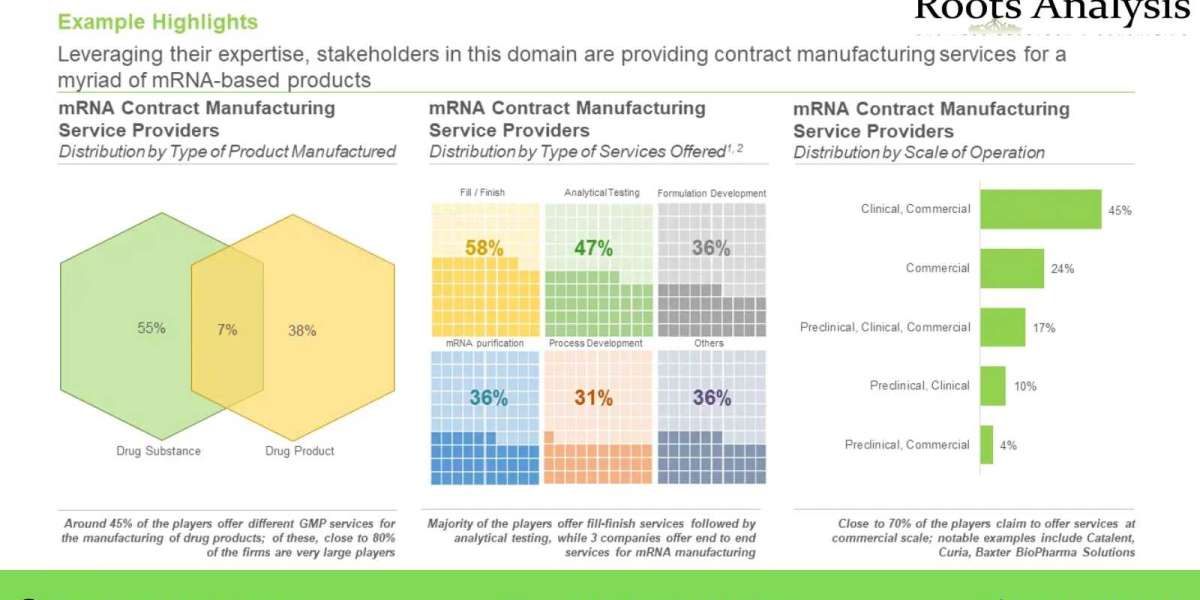
Players Engaged in mRNA Synthesis and Manufacturing Domain
Presently, mRNA synthesis and manufacturing service providers landscape features a mix of over 70 large, mid-sized and small companies, which claim to have the required expertise to offer various services for the synthesis and manufacturing of mRNAs across different scales of operations, worldwide. In addition to this, more than 95 mRNA synthesis kits, that contain reagents for the synthesis of research grade mRNAs, are currently available in the market. Recent developments in this segment of the biopharmaceutical industry indicate that the service providers are upgrading their capabilities and infrastructure to accommodate the current and anticipated demand for this novel class of biologics.
Concluding Remarks
Currently, dozens of preclinical and clinical reports demonstrating the efficacy of these platforms have been published in the last two years alone. However, owing to the several challenges associated with the production of mRNA-based therapeutics / vaccines, majority of the pharmaceutical companies prefer to outsource their manufacturing operations. In the foreseen future, as more of such RNA-based leads mature and move into the clinic and / or get commercialized, we anticipate the mRNA synthesis market to witness healthy growth.
For additional details, please visit
https://www.rootsanalysis.com/blog/mrna-synthesis-and-manufacturing/ or email [email protected]
You may also be interested in the following titles:
- Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
- 4D Bioprinting Market : Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
[email protected]