Further, it has been around three years since the COVID-19 pandemic hit the world, and yet, shows no signs of disappearing. An article published in The Lancet journal reported that COVID-19 pandemic caused death of over 18 million people in 2020 alone. In addition, it has been reported that human immunodeficiency virus (HIV), one of the leading viral diseases is anticipated to be the cause for 6.5 million deaths globally, by 2030.
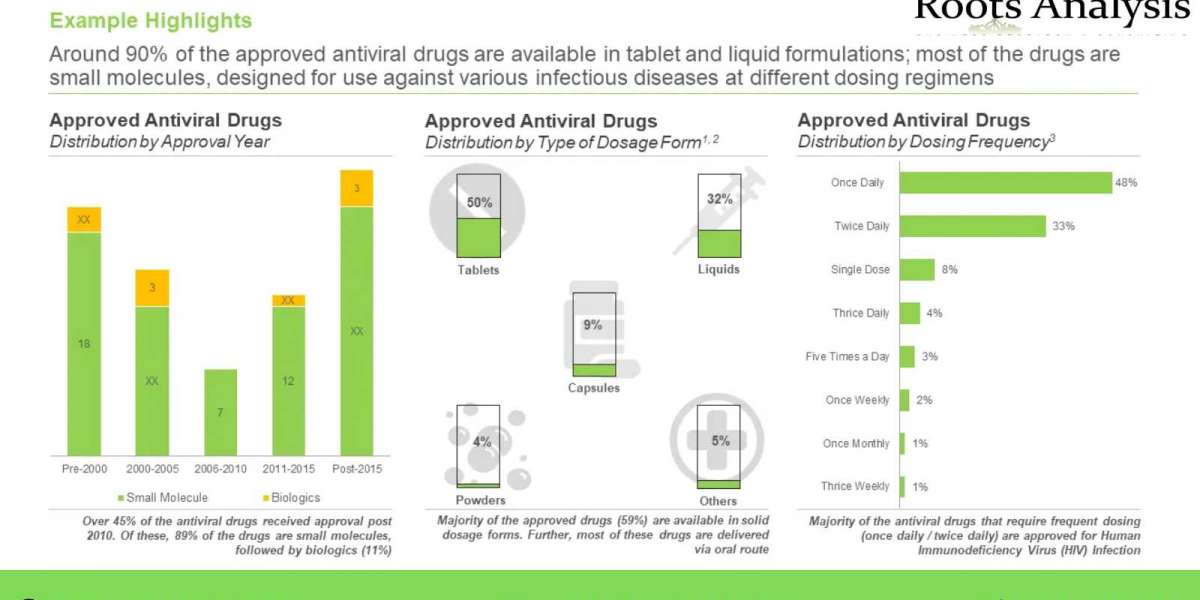
It is important to note that, currently approved antiviral drugs are capable of targeting only 10 of the 220 known viruses, responsible for causing multiple life-threatening infectious diseases in humans. The COVID-19 pandemic exposed the lack of effective antiviral therapeutics that can be used for the treatment of re-emerging or emerging viral diseases; this has also opened window of opportunities for the research and development of novel antiviral drugs that can be effective against multiple viruses.
The antiviral therapeutic development is fraught with several challenges, including emergence of drug resistance in viruses (as viruses can frequently mutate their genome) and concerns related to drug safety and efficacy. In order to address the aforementioned concerns, several industry stakeholders have been actively leading the RD efforts for the development of effective antiviral therapeutics against the known viral targets.
Clinical-stage Antiviral Drugs: Current Market Landscape
Presently, around 430 drug candidates are being investigated in various phases of clinical trials for the treatment of multiple infectious diseases; majority of the drugs in trials are small molecules and are being evaluated as monotherapies.
Antiviral Drugs: Current Developer Landscape
Currently, around 400 players are engaged in the development / distribution of antiviral drugs across the globe. Of these, 27 players have approved antiviral drugs. Although players that provide approved antiviral drugs are present across the globe, most of them are headquartered in North America, followed by those headquartered in Europe. Further, around 370 players are currently evaluating a number of antiviral drugs in trials against various viral diseases. The market landscape is highly fragmented, featuring the presence of both new entrants and well-established players; majority of these developers are based in developed geographies.
The Increasing Interest in this Field is also Reflected in Recent Partnership Activity
Since 2019, partnership activity within the antiviral drugs domain has increased with an annualized growth rate of 140%. Clinical trial agreements have captured a substantial share in the partnerships related to antiviral drugs, during the period of 2019-2022. This was followed by RD agreements, which have captured a significant share of the partnerships inked in this domain. Further, the maximum number of partnerships in this domain was observed in 2020, followed by those signed in 2021. This rise in partnership activity in the last two years can be attributed to the increasing demand of antiviral drugs to meet the drug supply demand amidst the COVID-19 pandemic.
Likely Growth of the Antiviral Drugs Market
During our research, we estimated the market under conservative, base and optimistic scenarios. As per the base case forecast scenario, market for antiviral drugs is estimated to be, growing at an annualized rate of 6.3%, in the given time period. The opportunity is likely to be well distributed across types of mechanism of actions, target indications, type of drug targets, type of therapies and key geographical regions.
Conclusion
Presently, more than 375 companies are engaged in the antiviral drug development of over 420 early and late-stage antiviral drugs, worldwide. These stakeholders have established multiple partnerships and collaborations in order to advance the development of various pipeline candidates. Moreover, in order to fund product development initiatives in this domain, various private and public sector investors have invested around USD 5.5 billion across multiple instances. Driven by the increasing demand for safe and effective antivirals, ongoing pace of innovation in this field, recent partnership activity and sufficient financial support from investors, the antiviral drugs market is anticipated to witness substantial growth in the mid to long-term.
For additional details, please visit
https://www.rootsanalysis.com/blog/antiviral-drugs-development/ or email [email protected]
You may also be interested in the following titles:
- Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
- AI-based Digital Pathology / AI Pathology Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
[email protected]