Further, CAR-T cell therapies, relatively recent addition to the gamut of anticancer interventions, has demonstrated significant promise. Overall, this highly specific and promising form of CAR-T cell therapy treatment, which harnesses the versatile effector machinery of the human immune system, has revolutionized cancer treatment, globally. Given the consistent increase in number of cell therapies being developed and launched, this upcoming therapeutic segment is on its way to becoming one of the highest valued markets within the biopharmaceutical industry.
At present, more than 5 CAR-T therapies have been approved for several hematological malignancies, including KYMRIAH® (Novartis), YESCARTA® (Gilead Sciences), TECARTUS™ (Gilead Sciences), Breyanzi® (Bristol Myers Squibb), Abecma™ (Bristol Myers Squibb) and CARVYKTI™ (Janssen Biotech / Legend Biotech). In fact, more than 170 companies are engaged in the development of over 970 early and late-stage CAR-T therapies, worldwide. Moreover, several promising leads are anticipated to be commercially launched over the coming decade, following which the market is projected to grow at a substantial pace.
Over 6,500 patents related to CAR-T cell therapies have been recently filed / granted, demonstrating the continued innovation in this domain. In addition, more than 260 collaborations have been inked between several industry / academic stakeholders in order to advance the development of various pipeline candidates. To fund product development initiatives, capital investments worth more than USD 24 billion have been made by various private and public sector investors, in the last few years. Driven by the ongoing pace of innovation in this field, sufficient financial support from investors and encouraging clinical trial results, the CAR-T cell therapies market is likely to witness significant growth in the foreseen future.
CAR-T Cell Therapies, With Over 970 Preclinical / Clinical Candidates, Represent One of The Most Active Segments Of The Pharmaceutical Domain
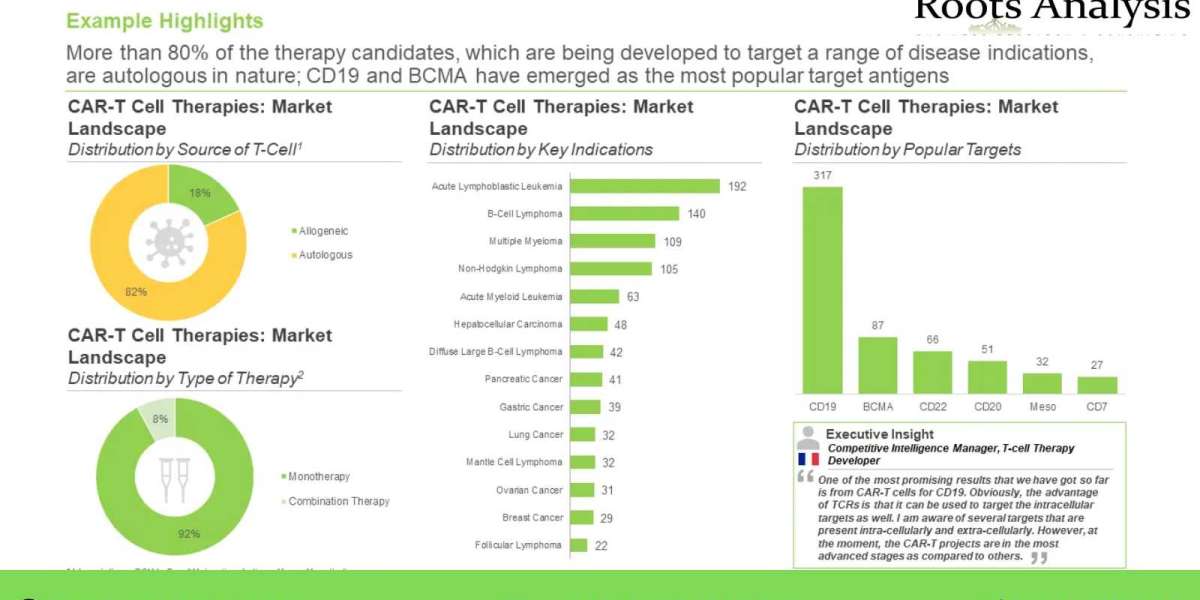
Majority (82%) of the CAR-T cell therapies are being developed using autologous T-cells, followed by those using allogeneic cells, derived from the blood of healthy donors. It is worth highlighting that majority of these CAR-T therapies are monotherapies (92%) followed by combination therapies. Additionally, most of the therapies being developed for the treatment of acute lymphoblastic leukemia, B-cell lymphoma, multiple myeloma, non-Hodgkin lymphoma and acute myeloid leukemia using several target antigens which includes CD19, BCMA, CD22, CD20, Meso, and CD7.
A Rise In Partnerships, In The Recent Past, Involving Both International And Indigenous Stakeholders, Validate The Growing Interest In This Domain; Maximum Number Of Such Deals Were Signed In 2021
Since 2015, the partnership activity in this domain has increased significantly. Given that most of the products are commercially available, in recent years, numerous agreements for product evaluation have been inked. Majority of partnerships are licensing agreements followed by RD agreements (21%); most of the licensing deals (68%) are focused on technology licensing for the development of CAR-T therapies.
Several Investors, Having Realized the Opportunity Within This Upcoming Segment, Have Invested Close To USD 25 Billion
More than 65% of the total funding amount was invested in the last five years. Specifically, in 2022 so far, companies engaged in this domain have collectively raised more than USD 7 Billion, majority of the funding was acquired through venture capital rounds, other equity financing elements and secondary offerings.
Over 6,500 Patents Related to Car-T Therapies Have Been Recently Filed / Granted, Demonstrating The Continued Innovation In This Domain
Majority of the patents related to CAR-T cell therapies have been filed by non-industry players; more than 1,400 such patents were filed in 2021 alone. Additionally, most of the patents in this domain are patent applications (84%), followed by granted patents (13%) and Others (3%).
The Projected Opportunity for Car-T Cell Therapies Is Likely to Be Well Distributed Across Different Segments And Is Estimated To Grow At An Annualized Rate Of 20%, Till 2035
The global CAR-T cell therapy market is estimated to be worth USD XX billion in 2022, and this value is projected to reach around USD XX billion in 2035, growing at a CAGR of 20%, during the period 2022-2035. In the long-term, the overall projected opportunity is likely to be well distributed across key market segments, including target indications, target antigen, key players and geographical regions.
For additional details, please visit
https://www.rootsanalysis.com/blog/car-t-cell-therapies-addressing-key-unmet/ or email [email protected]
You may also be interested in the following titles:
- 4D Bioprinting Market, 2023-2035
- Non-Viral / Intracellular Drug Delivery Systems Market, 2023-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415