London
Roots Analysis has announced the addition of “Adeno-Associated Viral Vector Market, 2022-2035” report to its list of offerings.
Owing to their unique biology, simple structure and lack of disease correlation, the adeno-associated viral vectors have garnered significant interest within the medical community. In fact, various adeno viral vector based therapies have been developed for the treatment of multiple indications, including retinitis pigmentosa, cystic fibrosis and Duchenne muscular dystrophy. As a result, the demand for such vectors have increased tremendously, thereby creating lucrative opportunities for the players engaged in the adeno-associated viral vector market. Considering the prevalent trends and projected opportunity associated with the overall adeno-associated viral vector / AAV vector domain, we believe that the market is anticipated to witness substantial growth in the foreseen future.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/adeno-associated-viral-vector-market/request-sample.html
Key Market Insights
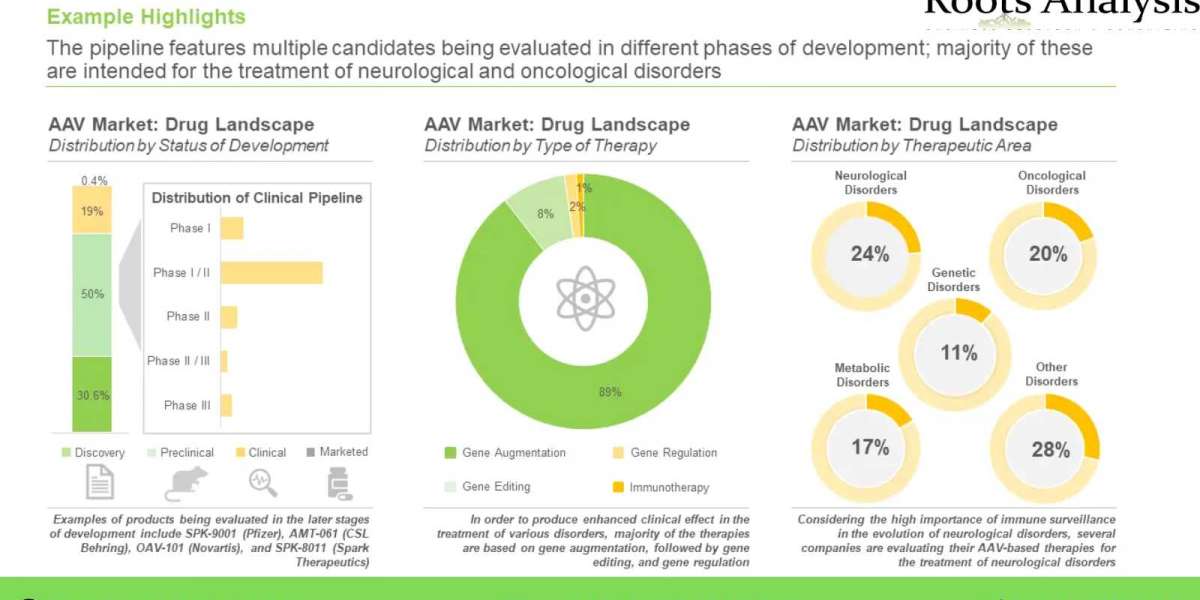
More than 550 adeno-associated viral vector-based therapies have been / are being evaluated across different stages of development for the treatment of various disorders
Close to 20% of the pipeline candidates are under clinical evaluation; majority (26%) of these candidates are being developed for the treatment of neurological disorders, followed by those being evaluated for the treatment of ophthalmic disorders (21%).
80+ industry stakeholders claim to have the required capability to manufacture adeno-associated viral vectors
The market is currently dominated by the presence of various mid-sized industry players (53%); of these 65% of the players possess in-house manufacturing capabilities, whereas 44% of players are contract manufacturers.
Over 30 players are involved in the development of adeno-associated vector platforms
More than 90% of these players offer their technologies for gene therapy, followed by those offering platforms for cell therapy (23%). It is worth mentioning that two technologies can manufacture vectors at all scales of operation, including preclinical, clinical and laboratory.
More than 155 clinical trials have been registered for the evaluation of adeno-associated viral vector-based therapies, worldwide
The clinical research activity, in terms of number of trials registered, is reported to have increased at a CAGR of 20%, since 2010. Of the total number of trials registered, close to 25% have already been completed, while 46% of the studies are actively recruiting participants.
Partnership activity within this domain has increased at a CAGR of ~50%, between 2017 and 2021
More than 60% of the reported deals were established since 2020, with the maximum activity (~50%) being reported in 2021. Majority of the partnerships were established for product development (30%), followed by agreements inked for research and development (15%).
Over 4,370 patents have been filed / granted related to adeno-associated viral vectors, since 2017
Close to 35% of these patents were filed / granted in North America, followed by Asia-Pacific (32%). It is worth highlighting that, in addition to the industry players, various non-industry / academic players have also filed patents related to adeno-associated viral vectors; these include University of Pennsylvania, University of Florida and the Research Institute of Nationwide Children's Hospital.
More than 50 start-ups have emerged in the last 10 years in the adeno-associated viral vector domain
Close to 60% of the start-ups have been established in North America. Among these, more than 95% of the firms are based in the US. This is followed by Europe, accounting for more than 30% of the start-ups focused on adeno-associated viral vectors.
North America is anticipated to capture larger share of the adeno-associated viral vector market by 2035
The market will be primarily driven by the manufacturers developing therapies for the treatment of oncological disorders (20%). In addition, by 2035, majority market share of the adeno-associated viral vector-based therapies is anticipated to be dominated by commercial scale of operation (46%), followed by preclinical (40%) and clinical (14%).
For additional details, please visit https://www.rootsanalysis.com/reports/adeno-associated-viral-vector-market.html or email [email protected]
You may also be interested in the following titles:
- Viral Vector Manufacturing, Non-Viral Vector Manufacturing and Gene Therapy Manufacturing Market (5th Edition): Industry Trends and Global Forecast, 2022-2035
- Gene Therapy Market (5th Edition): Industry Trends and Global Forecast, 2022-2035
Contact:
Ben Johnson
+1 (415) 800 3415