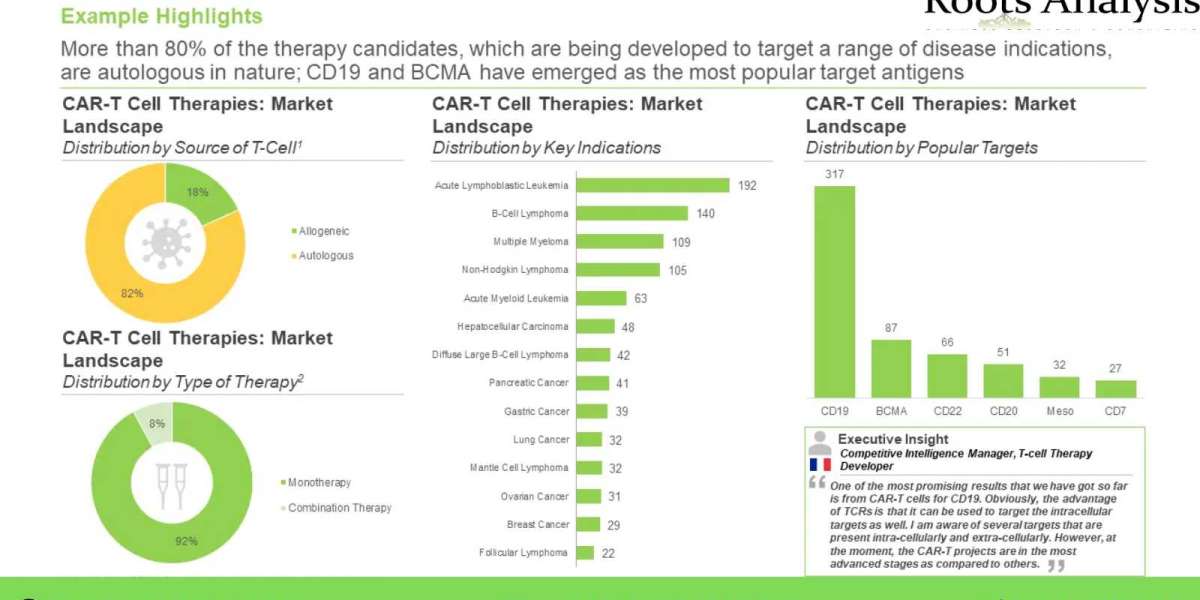
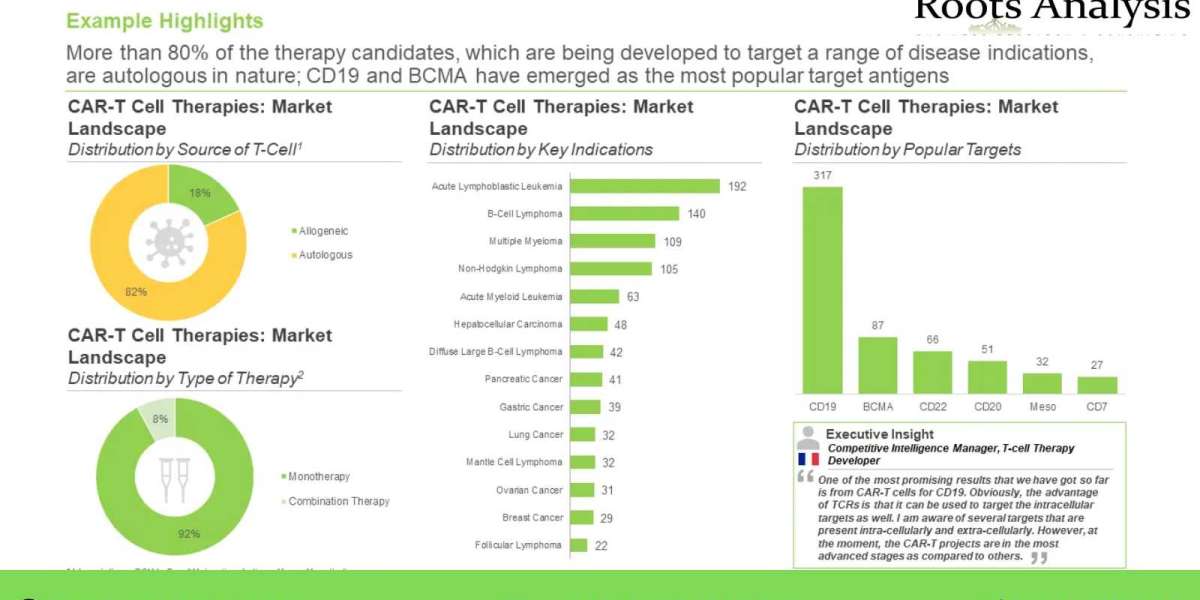
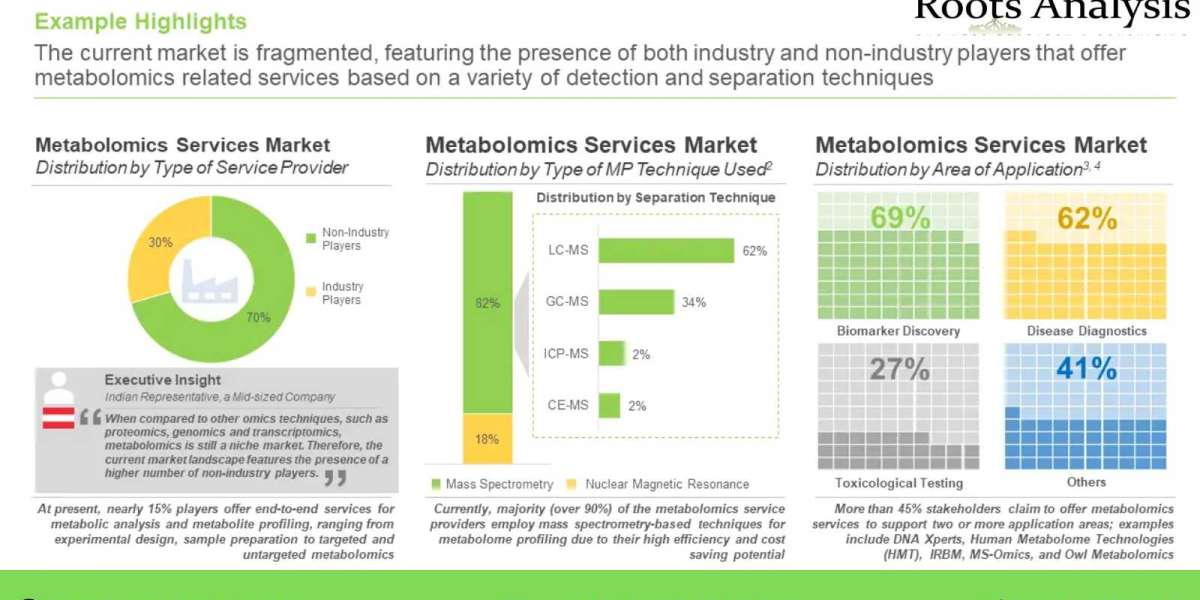
Further, CAR-T cell therapy, a relatively recent addition to the gamut of anticancer interventions, has demonstrated significant promise. Overall, this highly specific and promising form of CAR-T cell therapy treatment, which harnesses the versatile effector machinery of the human immune system, has revolutionized cancer treatment, globally. Given the consistent increase in number of cell therapies being developed and launched, this upcoming therapeutic segment is on its way to becoming one of the highest valued markets within the biopharmaceutical industry.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/269/request-sample.html
At present, more than 5 CAR-T therapies have been approved for several hematological malignancies, including KYMRIAH® (Novartis), YESCARTA® (Gilead Sciences), TECARTUS™ (Gilead Sciences), Breyanzi® (Bristol Myers Squibb), Abecma™ (Bristol Myers Squibb) and CARVYKTI™ (Janssen Biotech / Legend Biotech). In fact, more than 170 companies are engaged in the development of over 970 early and late-stage CAR-T therapies, worldwide. Moreover, several promising leads are anticipated to be commercially launched over the coming decade, following which the market is projected to grow at a substantial pace. Over 6,500 patents related to CAR-T cell therapies have been recently filed / granted, demonstrating the continued innovation in this domain. In addition, more than 260 collaborations have been inked between several industry / academic stakeholders in order to advance the development of various pipeline candidates. To fund product development initiatives, capital investments worth more than USD 24 billion have been made by various private and public sector investors, in the last few years. Driven by the ongoing pace of innovation in this field, sufficient financial support from investors and encouraging clinical trial results, the CAR-T cell therapy market is likely to witness significant growth in the foreseen future.
For additional details, please visit https://www.rootsanalysis.com/reports/view_document/car-t-therapies-market/269.html or email [email protected]
You may also be interested in the following titles:
- Targeted protein degradation market, 2022-2035
- Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091