Key Market Insights
- With over 195 candidates under development, mRNA therapeutics and mRNA Vaccines Market currently represents one of the fastest growing drug classes in the pharmaceutical market
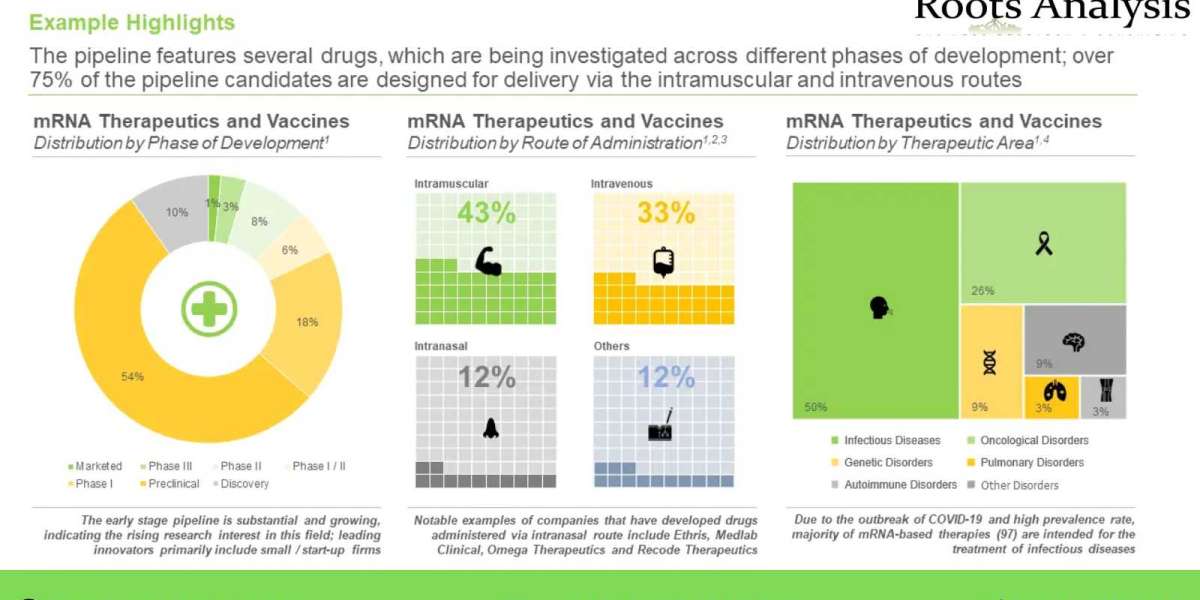
- The pipeline features several drugs, which are being investigated across different phases of development; over 75% of the pipeline candidates are designed for delivery via the intramuscular and intravenous routes
- Stakeholders are actively upgrading their existing capabilities to further enhance their respective product pipeline and comply to the evolving industry benchmarks
- Presently, around 17 start-ups are driving innovation in this domain; various RD initiatives have been undertaken by these players over the last few years
- More than 345,000 patients have been recruited / enrolled in clinical trials registered for this novel class of therapies, across different geographies
- Over 430 patents related to mRNA therapeutics and vaccines have recently been filed / granted by industry and non-industry stakeholders, indicating the widening intellectual capital in this domain
- The growing interest is evident from the rise in partnership activity, since 2021; clinical trial agreements has emerged as the most common type of partnership model adopted by drug developers
- Several investors, having realized the opportunity within this upcoming segment, have invested over USD 11 billion across various funding rounds in the past nine years
- Big pharma players have undertaken several initiatives, ranging from proprietary product development to strategic investments, to tap the lucrative opportunity in this rapidly growing market
- The market’s evolution is likely to be driven by the rising need for novel drug candidates; we expect the future opportunity to be well distributed across various routes of administration, therapeutic areas and key regions
Table of Contents
PREFACE
1.1. Scope of the Report
1.2. Market Segmentation
1.3. Research Methodology
1.4. Key Questions Answered
1.5. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. mRNA Therapeutics
3.2.1. Advantages of mRNA Therapeutics
3.2.2. Key Applications of mRNA Therapeutics
3.3. mRNA Vaccines
3.4. mRNA Delivery Routes
3.5. mRNA Delivery Strategies
3.6. Key Challenges Associated with mRNA Therapeutics and Vaccines
3.7. Future Perspectives
- MARKET OVERVIEW
4.1. Chapter Overview
4.2. mRNA Therapeutics and Vaccines: Overall Market Overview
- COMPETITIVE LANDSCAPE
5.1. mRNA Therapeutics and Vaccines: List of Developers
- COMPANY COMPETITIVENESS ANALYSIS
6.1. Chapter Overview
6.2. Key Assumptions and Parameters
6.3. Methodology
6.4. mRNA Therapeutics and Vaccines Developers: Company Competitive Analysis
6.5. mRNA Therapeutics and Vaccines Developers based in North America
6.6. mRNA Therapeutics and Vaccines Developers based in Europe
6.7. mRNA Therapeutics and Vaccines Developers based in Asia-Pacific and Rest of the World
- DRUG PROFILES
7.1. Chapter Overview
7.2. BNT162b2 (BioNTech / Pfizer)
7.3. GEMCOVAC-19 (Gennova)
7.4. mRNA-1273 (Moderna)
7.5. LUNAR-COV19 (Arcturus Therapeutics)
7.6. CVnCOV (CureVac)
7.7. mRNA-1010 (Moderna)
7.8. mRNA-1345 (Moderna)
7.9. mRNA-1647 (Moderna)
7.10. ARCoV (Walvax)
- BIG PHARMA INITIATIVES
8.1. Chapter Overview
8.2. Scope and Methodology
8.3. mRNA Related Initiatives of Big Pharmaceutical Players
8.4. Benchmark Analysis of Big Pharmaceutical Players
- START-UP HEALTH INDEXING
9.1. Chapter Overview
9.2. Start-ups focused on mRNA Therapeutics and Vaccines
9.3. Benchmarking of Start-ups
9.4. Startup-Health Indexing
- CLINICAL TRIAL ANALYSIS
10.1. Chapter Overview
10.2. Scope and Methodology
10.3. mRNA Therapeutics and Vaccines: Clinical Trial Analysis
- PARTNERSHIPS AND COLLABORATIONS
11.1. Chapter Overview
11.2. Partnership Models
11.3. mRNA Therapeutics and Vaccines: Partnerships and Collaborations
- FUNDING AND INVESTMENT ANALYSIS
12.1. Chapter Overview
12.2. Types of Funding
12.3. mRNA Therapeutics and Vaccines: Funding and Investment
- PATENT ANALYSIS
13.1. Chapter Overview
13.2. Scope and Methodology
13.3. mRNA Therapeutics and Vaccines: Patent Analysis
13.4. mRNA Therapeutics and Vaccines: Patent Benchmarking Analysis
- MARKET FORECAST AND OPPORTUNITY ANALYSIS
14.1. Chapter Overview
14.2. Key Assumptions and Methodology
14.3. Global mRNA Therapeutics and Vaccines Market, 2022-2035
14.4. mRNA Therapeutics and Vaccines Market: Value Creation Analysis
14.5. mRNA Therapeutics and Vaccines Market: Product-wise Sales Forecasts
- CONCLUSION
- EXECUTIVE INSIGHTS
16.1. Chapter Overview
16.2. eTheRNA Immunotherapies
- APPENDIX I: TABULATED DATA
- APPENDIX II: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/mrna-therapeutics-and-vaccines-market.html
You may also be interested in the following titles:
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415